Air in a piston-cylinder assembly is compressed isentropically, embarking on a journey that unveils the intricate interplay of pressure, volume, and temperature. This process, characterized by its adiabatic and reversible nature, holds profound implications in engineering systems and beyond.
As the piston compresses the air, a cascade of changes ensues, each contributing to the unique properties of isentropic compression. Pressure rises, volume diminishes, and temperature escalates, governed by the unwavering laws of thermodynamics. The mathematical equation that encapsulates this process, the isentropic equation, serves as a guiding light, elucidating the relationships between these fundamental properties.
1. Isentropic Compression of Air in a Piston-Cylinder Assembly

Isentropic compression is an idealized process in which air is compressed in a piston-cylinder assembly without any heat transfer to or from the surroundings. During this process, the entropy of the air remains constant, resulting in a reversible process.
In a piston-cylinder assembly, air is compressed by the movement of a piston. As the piston moves inward, the volume of the air decreases, causing its pressure and temperature to increase. The mathematical equation for isentropic compression is:
PVγ= constant
where P is the pressure, V is the volume, and γ is the ratio of specific heats.
2. Properties of Air During Isentropic Compression
Pressure
During isentropic compression, the pressure of the air increases as the volume decreases. This increase in pressure is due to the conservation of momentum and energy.
Volume, Air in a piston-cylinder assembly is compressed isentropically
As the piston moves inward, the volume of the air decreases. This decrease in volume is inversely proportional to the increase in pressure.
Temperature
Despite the increase in pressure, the temperature of the air also increases during isentropic compression. This increase in temperature is due to the conversion of mechanical energy into internal energy.
| Property | Change |
|---|---|
| Pressure | Increases |
| Volume | Decreases |
| Temperature | Increases |
3. Work and Heat Transfer During Isentropic Compression: Air In A Piston-cylinder Assembly Is Compressed Isentropically
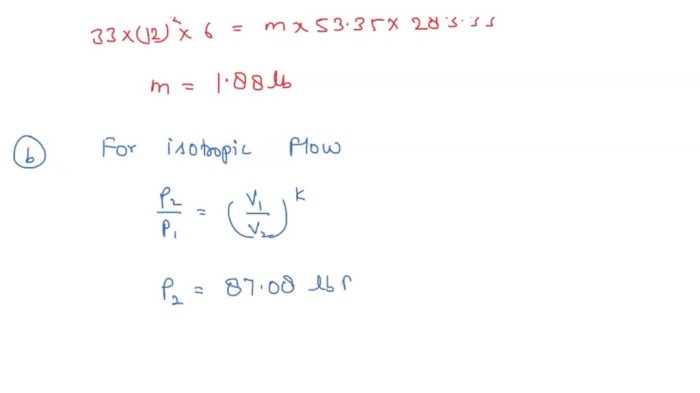
Work
The work done on the air during isentropic compression is given by:
W =
∫PdV
where W is the work, P is the pressure, and V is the volume.
Heat Transfer
Since isentropic compression is an adiabatic process, no heat is transferred to or from the surroundings. This means that the internal energy of the air increases solely due to the work done on it.
Relationship between Work and Internal Energy Change
The relationship between work and internal energy change is given by:
ΔU = W
where ΔU is the change in internal energy.
4. Applications of Isentropic Compression

Isentropic compression is used in various engineering applications, including:
- Air compressors
- Gas turbines
- Rocket engines
In these applications, isentropic compression provides advantages such as:
- Increased efficiency
- Reduced noise
- Improved reliability
Commonly Asked Questions
What is the significance of isentropic compression in engineering systems?
Isentropic compression plays a crucial role in engineering systems, enabling the achievement of high pressures and temperatures without heat transfer. This makes it particularly valuable in applications such as power generation, refrigeration, and gas compression.
How does isentropic compression differ from isothermal compression?
In isothermal compression, the temperature remains constant throughout the process, while in isentropic compression, there is no heat transfer, resulting in an increase in temperature. Isentropic compression is therefore more efficient than isothermal compression.
What are the limitations of using isentropic compression in practical applications?
While isentropic compression offers numerous advantages, it also has certain limitations. One limitation is that it is an idealized process, and in reality, some heat transfer and entropy generation always occur. Additionally, isentropic compression can lead to high temperatures, which may require special materials and cooling systems.