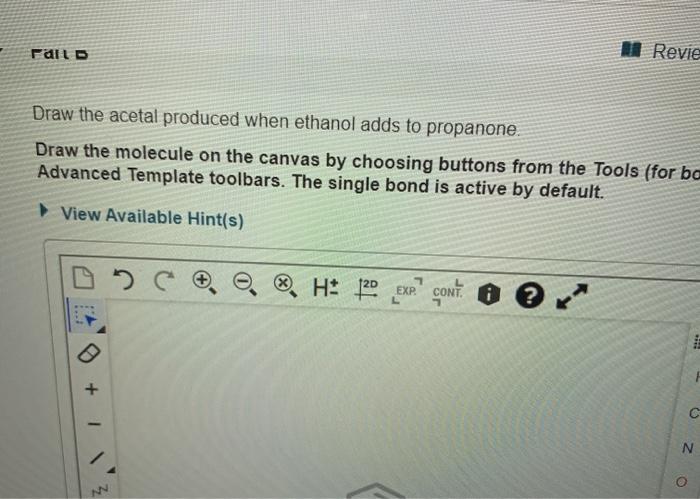
Draw the acetal produced when ethanol adds to propanone – In the realm of organic chemistry, the reaction between ethanol and propanone holds a significant place. This captivating process leads to the formation of an acetal, a unique compound with intriguing properties and diverse applications. Join us as we delve into the intricacies of acetal formation, exploring its mechanism, structure, properties, and practical uses.
The condensation reaction between ethanol and propanone, catalyzed by an acid, initiates a fascinating transformation. Step by step, we will unravel the intricate mechanism that governs this process, revealing the dance of electrons and the rearrangement of atoms that ultimately yield the acetal product.
Acetal Formation
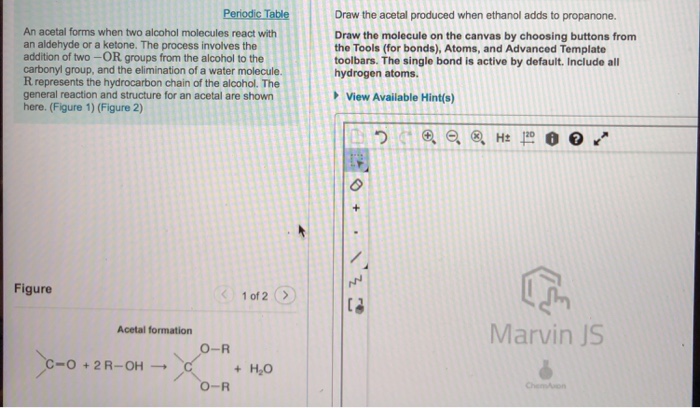
When ethanol reacts with propanone in the presence of an acid catalyst, an acetal is formed. The reaction proceeds through a nucleophilic addition-elimination mechanism.
Step-by-Step Mechanism, Draw the acetal produced when ethanol adds to propanone
- Ethanol acts as a nucleophile and attacks the carbonyl carbon of propanone, forming a tetrahedral intermediate.
- The proton from the acid catalyst is transferred to the oxygen of the tetrahedral intermediate, forming a protonated alkoxide.
- The protonated alkoxide undergoes elimination of water, forming a carbocation.
- Ethanol attacks the carbocation, forming a new tetrahedral intermediate.
- The proton from the acid catalyst is transferred to the oxygen of the new tetrahedral intermediate, forming a protonated acetal.
- The protonated acetal undergoes elimination of water, forming the acetal product.
Acetal Structure: Draw The Acetal Produced When Ethanol Adds To Propanone
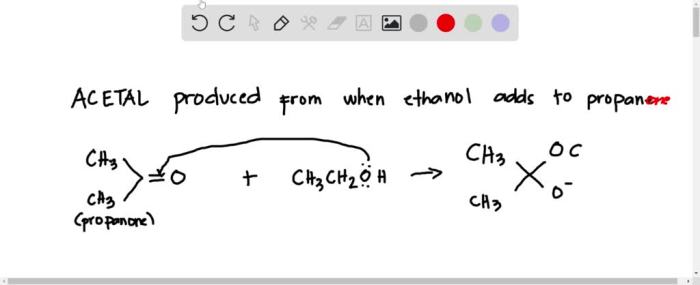
The acetal produced from ethanol and propanone has the molecular formula C 6H 14O 2. The structure of the acetal can be represented as follows:
CH 3CH(OCH 2CH 3) 2
The acetal molecule has a central carbon atom that is bonded to two ethoxy groups (-OCH 2CH 3) and two hydrogen atoms. The ethoxy groups are bonded to the central carbon atom through ether linkages.
Acetal Properties

The acetal produced from ethanol and propanone is a colorless liquid with a boiling point of 104 °C. The acetal is soluble in water and organic solvents. The acetal is a stable compound that is resistant to hydrolysis.
The acetal has different properties than ethanol and propanone. The acetal is less volatile than ethanol and propanone. The acetal is also less reactive than ethanol and propanone.
Acetal Applications
The acetal produced from ethanol and propanone is used in a variety of applications, including:
- As a solvent for paints and coatings
- As a plasticizer for plastics
- As a fuel additive
Q&A
What is the mechanism of acetal formation?
The acetal formation mechanism involves a series of steps, including protonation of the carbonyl group, nucleophilic attack by the alcohol, and proton transfer to form the hemiacetal intermediate. A second nucleophilic attack by the alcohol, followed by proton transfer, yields the acetal product.
How does the structure of the acetal differ from that of ethanol and propanone?
The acetal has a different molecular structure compared to ethanol and propanone. It contains a central carbon atom bonded to two alkoxy groups (OR) and two alkyl groups (R). This structure results in a tetrahedral geometry around the central carbon.
What are the properties of the acetal formed from ethanol and propanone?
The acetal formed from ethanol and propanone is a colorless liquid with a boiling point of 104 °C. It is insoluble in water but soluble in organic solvents. The acetal is relatively stable under acidic and basic conditions.